Immobilized CALB
CALB is immobilized by physical adsorption on the highly hydrophobic resin that is a macroporous, styrene/methacrylate polymer. Immobilized CALB is suitable for applications in organic solvents and solvent-free systems, and can be recycled and reused for much times in suitable conditions.
Product Code: SZ-CALB- IMMO100A, SZ-CALB- IMMO100B.
★Higher activity, higher chiral selectivity and higher stability.
★Better performance in the non-aqueous phases.
★Easily remove from reaction system, rapidly terminate reactions, and avoid protein residue in product.
★Can be recycled and reused to cut down the cost.
|
Activity |
≥10000PLU/g |
|
pH range for reaction |
5-9 |
|
Temperature range for reaction |
10-60℃ |
|
Appearance |
CALB-IMMO100-A: Light yellow to brown solid CALB-IMMO100-B: White to light brown solid |
|
Particle size |
300-500μm |
|
Loss on drying at 105℃ |
0.5%-3.0% |
|
Resin for immobilization |
Macroporous, styrene/methacrylate polymer |
|
Reaction solvent |
Water, organic solvent, etc., or without solvent. For the reaction in some organic solvents, 3% water can be added to improve the reaction effect |
|
Particle size |
CALB-IMMO100-A: 200-800 μm CALB-IMMO100-B: 400-1200 μm |
Unit definition: 1 unit corresponds to the synthesis of 1μmol per minute propyl laurate from lauric acid and 1-propanol at 60℃. The above CALB-IMMP100-A and CALB-IMMO100-B correspond to immobilized carriers with different particle sizes.
1. Reactor type
The immobilized enzyme is applicable to both kettle batch reactor and fixed bed continuous flow reactor. It should be noted to avoid crushing due to external force during feeding or filling.
2. Reaction pH, temperature and solvent
The immobilized enzyme should be add last, after other materials added and dissolved, and pH adjusted.
If the consumption of substrate or the formation of product will lead to the change of pH during the reaction, sufficient buffer should be added to the reaction system, or the pH should be monitored and adjusted during the reaction.
Within the temperature tolerance range of CALB (below 60 ℃), the conversion rate increased with the increase of temperature. In practical use, the reaction temperature should be selected according to the stability of the substrate or product.
Generally, the ester hydrolysis reaction is suitable in aqueous phase system, while the ester synthesis reaction is suitable in organic phase system. The organic solvent can be ethanol, tetrahydrofuran, n-hexane, n-heptane and toluene, or a suitable mixed solvent. For the reaction in some organic solvents, 3% water can be added to improve the reaction effect.
3. Reuse and service life of CALB
Under the appropriate reaction condition, CALB can be recovered and reused, and the specific application times vary with different projects.
If the recovered CALB is not reused continuously and needs to be stored after recovery, it needs to be washed and dried and sealed at 2-8 ℃.
After several rounds of reuse, if the reaction efficiency is slightly reduced, CALB can be added appropriately and continued to use. If the reaction efficiency is reduced seriously, it needs to be replaced.
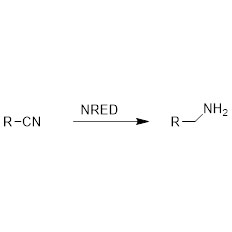
Example 1(Aminolysis)(1):
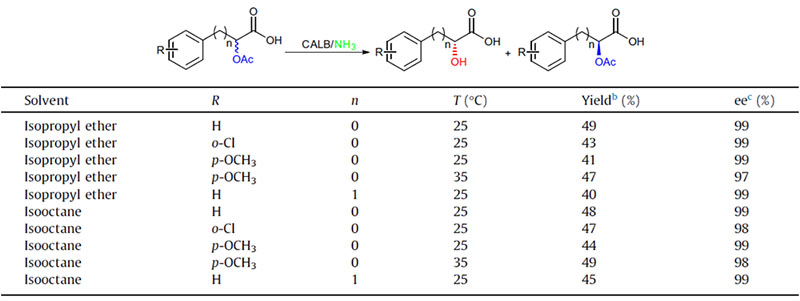
Example 2(Aminolysis) (2):
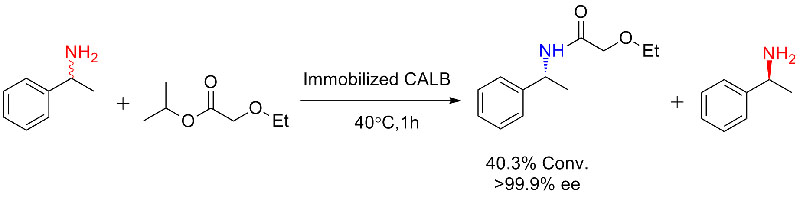
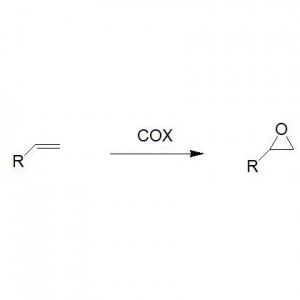
Example 3(Ring opening polyester synthesis) (3):
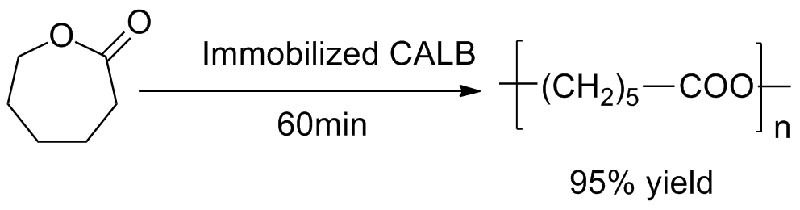
Example 4(Transesterification, regioselective of hydroxyl group) (4):
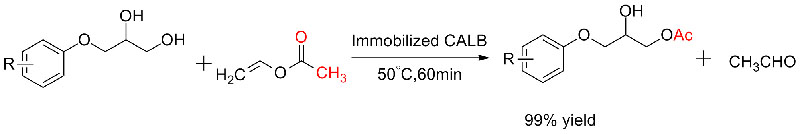
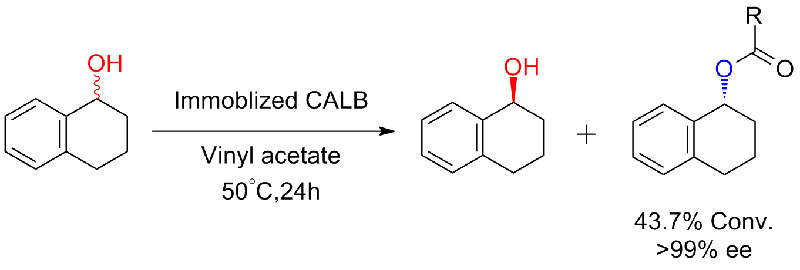
Example 5(Transesterification, kinetic resolution of racemic alcohols)(5):
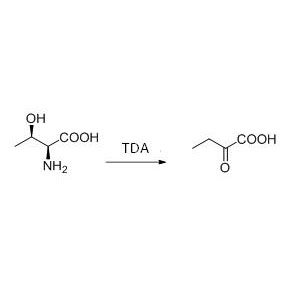
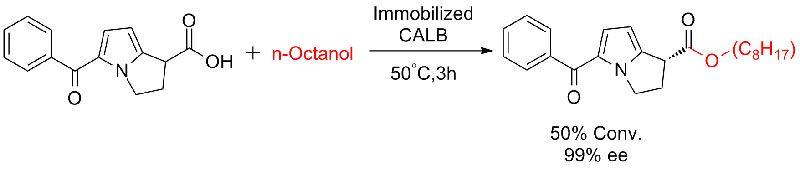
Example 6(Esterification, kinetic resolution of carboxylic acid) (6):
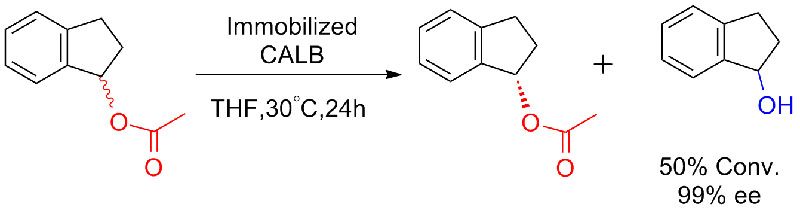
Example 7(Esterolysis, kinetic resolution) (7):
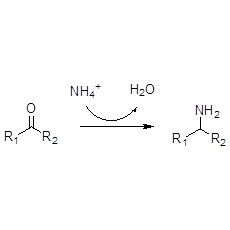
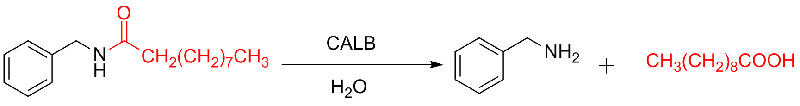
Example 8(Hydrolysis of amides) (8):
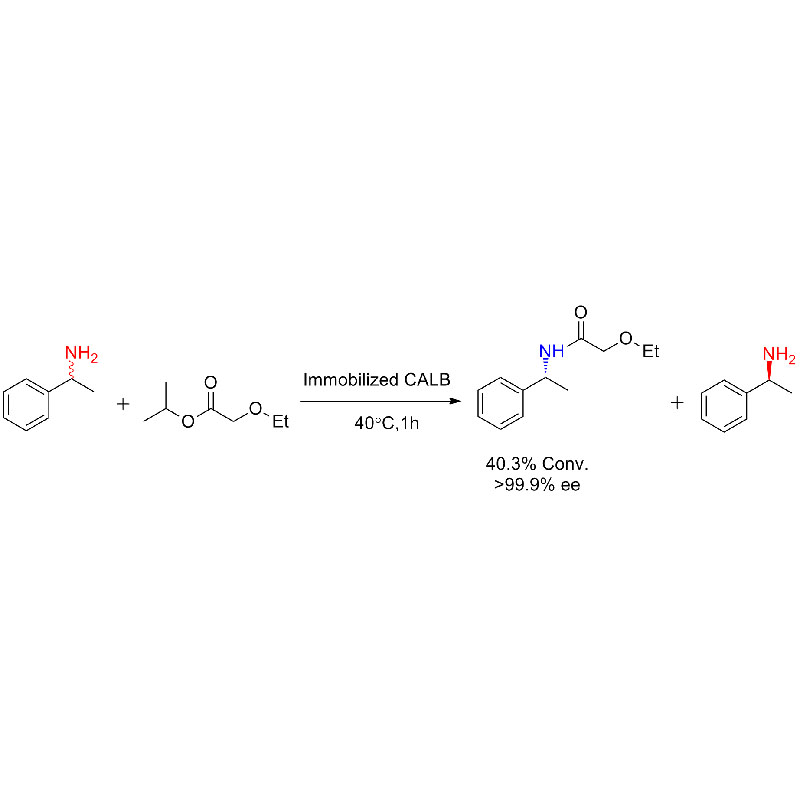
Example 9(Acylation of amines) (9):
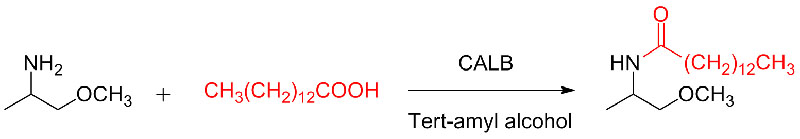

Example 10(Aza-Michael addition reaction) (10):
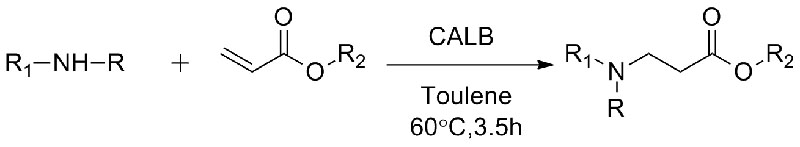
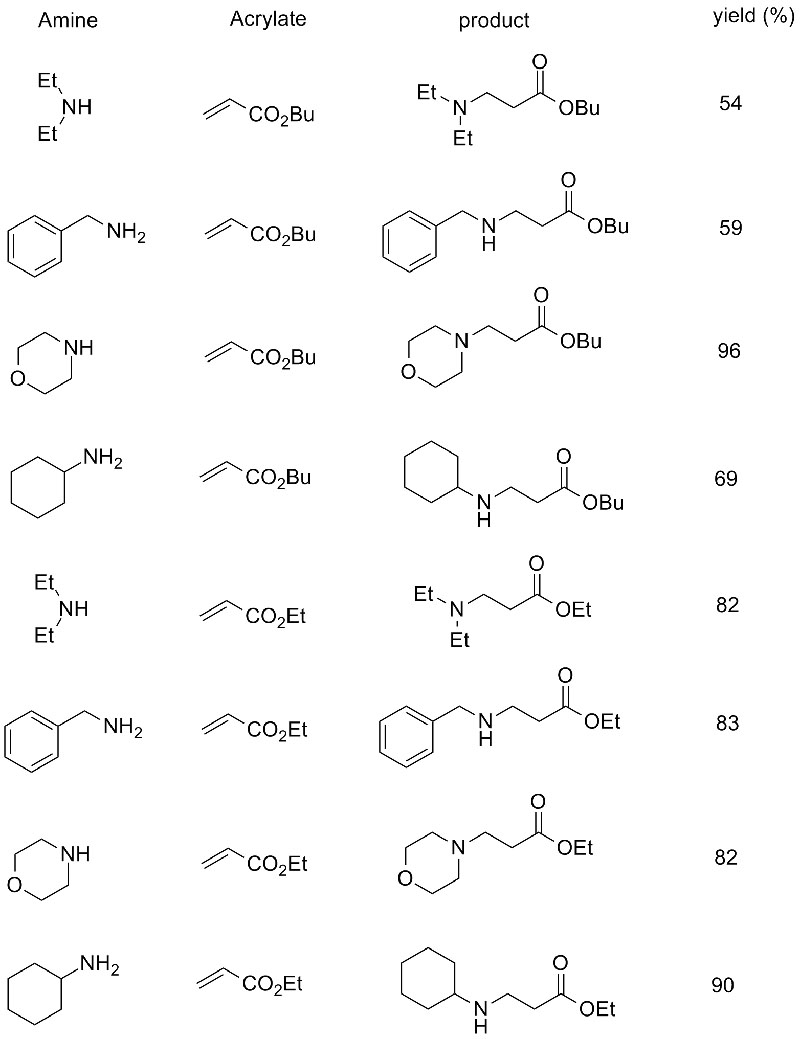
1. Chen S, Liu F, Zhang K, e tal. Tetrahedron Lett, 2016, 57: 5312-5314.
2. Olah M, Boros Z, anszky G H, e tal. Tetrahedron, 2016, 72: 7249-7255.
3. Nakaoki1 T, Mei Y, Miller L M, e tal. Ind. Biotechnol, 2005, 1(2):126-134.
4. Pawar S V, Yadav G D. J. Ind. Eng. Chem, 2015, 31: 335-342.
5. Kamble M P, Shinde S D, Yadav G D. J. Mol. Catal. B: Enzym, 2016, 132: 61-66.
6. Shinde S D, Yadav G D. Process Biochem, 2015, 50: 230-236.
7. Souza T C, Fonseca T S, Costa J A, e tal. J. Mol. Catal. B: Enzym, 2016, 130: 58-69.
8. Gavil´an A T, Castillo E, L´opez-Mungu´A. J. Mol. Catal. B: Enzym, 2006, 41: 136-140.
9. Joubioux F L, Henda Y B, Bridiau N, e tal. J. Mol. Catal. B: Enzym, 2013, 85-86: 193-199.
10. Dhake K P, Tambade P J, Singhal R S, e tal. Tetrahedron Lett, 2010, 51: 4455-4458.