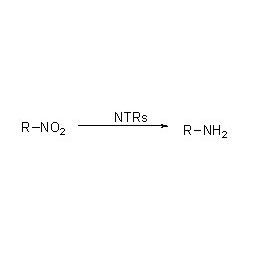
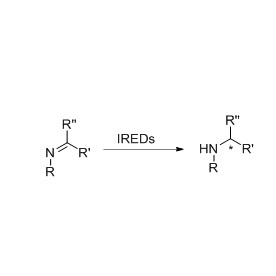
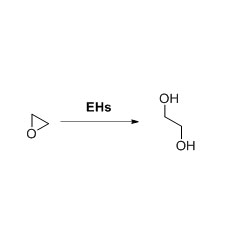
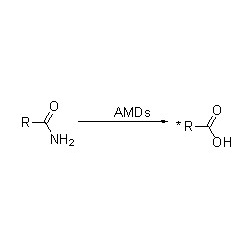
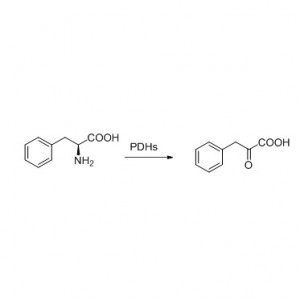
Transaminase (ATA)
Enzymes: Are macromolecular biological catalysts, most enzymes are proteins.
Transaminases: A class of enzymes that catalyze amino transferring between amino acids and keto acids. Transaminases are key biological enzymes in asymmetric synthesis and racemic resolution of chiral amines.
Aminotransferase can be divided into four classes according to sequence and structure: Ⅰ, Ⅱ, Ⅲ and Ⅳ. ω-aminotransferases belong to class II transaminases, commonly be used in the preparation of chiral amines and unnatural amino acids, such as β-amino acids.
ω-aminotransferases: In most cases, ω-transaminase refers to a class of enzymes, that catalytic ammonia transfer reactions without α-amino acid as substrate or product.
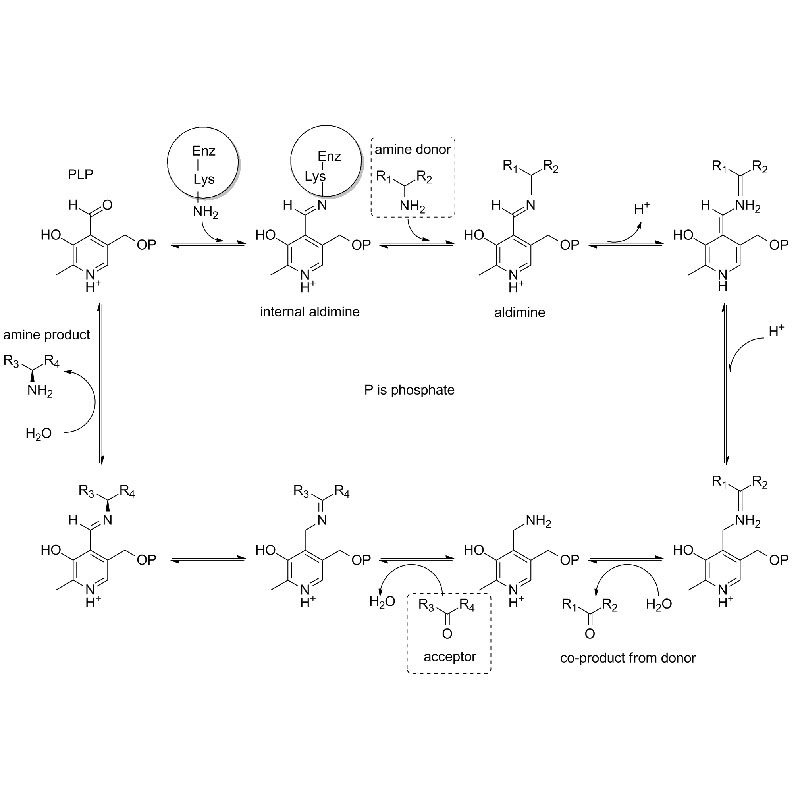
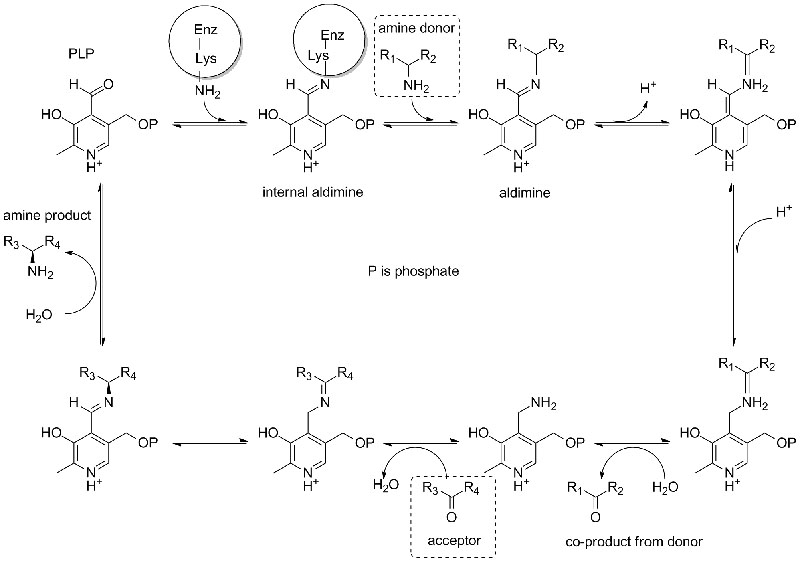
Catalytic mechanism:
| Enzymes | Product Code | Product Code |
| Enzyme Powder | ES-ATA-101~ ES-ATA-165 | a set of 65 ω-Transaminases, 50 mg each 65 items * 50mg / item, or other quantity |
| Screening Kit (SynKit) | ES-ATA-6500 | a set of 65 ω-Transaminases, 1 mg each 65 items * 1mg / item |
★ High substrate specificity.
★ Strong chiral selectivity.
★ High conversion efficiency.
★ Less by-products.
★ Mild reaction conditions.
★ Environmentally friendly.
➢ Enzyme screening should be carried out for specific substrates because of the substrate specificity, and get an enzyme that catalyzes the target substrate with best catalytic effect.
➢ Never contact with extreme conditions such as: high temperature, high/low pH and organic solvent with high concentration.
➢ Normally, the reaction system should include substrate, buffer solution, amino donor (such as amino acids and 1-phenyl ethylamine) or receptor (such as keto acids), coenzyme (PLP), cosolvent (such as DMSO).
➢ ATA should be added last into reaction system, after the pH and temperature been adjusted to the reaction condition.
➢ All kinds of ATA have various optimum reaction conditions, so each of them should be further studied individually.
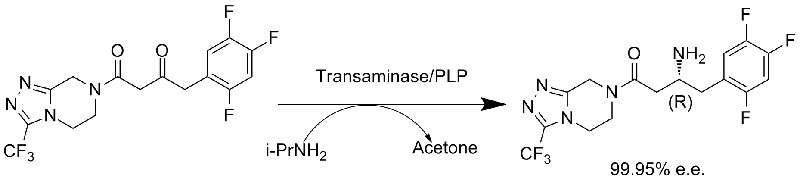
Example 1(synthesis of Sitagliptin, asymmetric synthesis) (1):
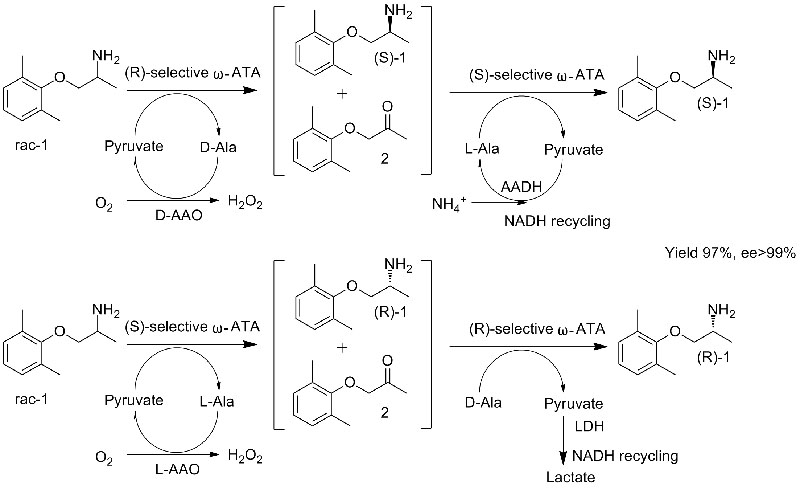
Example 2 (Mexiletine, Combination of kinetic resolution with asymmetric synthesis)(2):
1 Savile CK, Janey JM, Mundorff EC, et al. Science, 2010, 329(16), 305-309.
2 Koszelewski D, Pressnitz D, Clay D, et al. Organic letters, 2009,11(21):4810-4812.